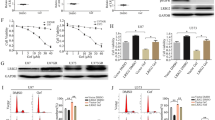
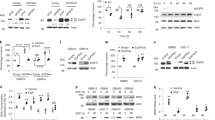
Abstract
In glioblastoma (GBM), the EGF receptor (EGFR) and Src family kinases (SFKs) contribute to an aggressive phenotype. EGFR may be targeted therapeutically; however, resistance to EGFR-targeting drugs such as Erlotinib and Gefitinib develops quickly. In many GBMs, a truncated form of the EGFR (EGFRvIII) is expressed. Although EGFRvIII is constitutively active and promotes cancer progression, its activity is attenuated compared with EGF-ligated wild-type EGFR, suggesting that EGFRvIII may function together with other signaling receptors in cancer cells to induce an aggressive phenotype. In this study, we demonstrate that in EGFRvIII-expressing GBM cells, the urokinase receptor (uPAR) functions as a major activator of SFKs, controlling phosphorylation of downstream targets, such as p130Cas and Tyr-845 in the EGFR in vitro and in vivo. When EGFRvIII expression in GBM cells was neutralized, either genetically or by treating the cells with Gefitinib, paradoxically, the cells demonstrated increased cell migration. The increase in cell migration was explained by a compensatory increase in expression of urokinase-type plasminogen activator, which activates uPAR-dependent cell signaling. GBM cells that were selected for their ability to grow in vivo in the absence of EGFRvIII also demonstrated increased cell migration, due to activation of the uPAR signaling system. The increase in GBM cell migration, induced by genetic or pharmacologic targeting of the EGFR, was blocked by Dasatinib, highlighting the central role of SFKs in uPAR-promoted cell migration. These results suggest that compensatory activation of uPAR-dependent cell signaling, in GBM cells treated with targeted therapeutics, may adversely affect the course of the disease by promoting cell migration, which may be associated with tumor progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Stettner MR, Wang W, Nabors LB, Bharara S, Flynn DC, Grammer JR et al. Lyn kinase activity is the predominant cellular SRC kinase activity in glioblastoma tumor cells. Cancer Res 2005; 65: 5535–5543.
Du J, Bernasconi P, Clauser KR, Mani DR, Finn SP, Beroukhim R et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol 2009; 27: 77–83.
Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D et al. Fyn and Src are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res 2009; 69: 6889–6898.
Ahluwalia MS, de Groot J, Liu WM, Gladson CL . Targeting Src in glioblastoma tumors and brain metastases: rationale and preclinical studies. Cancer Lett 2010; 298: 139–149.
Yeatman TJ . A renaissance for SRC. Nat Rev Cancer 2004; 4: 470–480.
Alper O, Bowden ET . Novel insights into c-Src. Curr Pharm Des 2005; 11: 1119–1130.
Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P . Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J 1999; 18: 2459–2471.
Felsenfeld DP, Schwartzberg PL, Venegas A, Tse R, Sheetz MP . Selective regulation of integrin-cytoskeleton interactions by the tyrosine kinase Src. Nat Cell Biol 1999; 1: 200–206.
Ma YC, Huang J, Ali S, Lowry W, Huang XY . Src tyrosine kinase is a novel directeffector of G proteins. Cell 2000; 102: 635–646.
Kubota Y, Tanaka T, Kitanaka A, Ohnishi H, Okutani Y, Waki M et al. Src transduces erythropoietin-induced differentiation signals through phosphatidylinositol 3-kinase. EMBO J 2001; 20: 5666–5677.
Servitja JM, Marinissen MJ, Sodhi A, Bustelo XR, Gutkind JS . Rac1 function is required for Src-induced transformation. Evidence of a role for Tiam1 and Vav2 in Rac activation by Src. J Biol Chem 2003; 278: 34339–34346.
Tice DA, Biscardi JS, Nickles AL, Parsons SJ . Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA 1999; 96: 1415–1420.
Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM . STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem 2003; 278: 1671–1679.
Jo M, Thomas K, Takimoto S, Gaultier A, Hsieh E, Lester R et al. Urokinase receptor primes cells to proliferate in response to epidermal growth factor. Oncogene 2007; 26: 2585–2594.
Hu J, Jo M, Cavenee WK, Furnari F, VandenBerg SR, Gonias SL . Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells. Proc Natl Acad Sci USA 2011; 108: 15984–15989.
Chumbalkar V, Latha K, Hwang Y, Maywald R, Hawley L, Sawaya R et al. Analysis of phosphotyrosine signaling in glioblastoma identifies STAT5 as a novel downstream target of DeltaEGFR. J Proteome Res 2011; 10: 1343–1352.
Boerner JL, Demory ML, Silva C, Parsons SJ . Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol 2004; 24: 7059–7071.
Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature 1985; 313: 144–147.
Schlegel J, Stumm G, Brandle K, Merdes A, Mechtersheimer G, Hynes NE et al. Amplification and differential expression of members of the erbB-gene family in human glioblastoma. J Neurooncol 1994; 22: 201–207.
Schlegel J, Merdes A, Stumm G, Albert FK, Forsting M, Hynes N et al. Amplification of the epidermal-growth-factor-receptor gene correlates with different growth behaviour in human glioblastoma. Int J Cancer 1994; 56: 72–77.
Sugawa N, Ekstrand AJ, James CD, Collins VP . Identical splicing of aberrant epidermal growth factor receptor transcripts from amplified rearranged genes in human glioblastomas. Proc Natl Acad Sci USA 1990; 87: 8602–8606.
Huang HS, Nagane M, Klingbeil CK, Lin H, Nishikawa R, Ji XD et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem 1997; 272: 2927–2935.
Blasi F, Carmeliet P . uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 2002; 3: 932–943.
Liu D, Aguirre Ghiso J, Estrada Y, Ossowski L . EGFR is a transducer of the urokinase receptor initiated signal that is required for in vivo growth of a human carcinoma. Cancer Cell 2002; 1: 445–457.
Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle UH, Majdic O et al. Urokinase plasminogen activator receptor, beta 2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med 1995; 181: 1381–1390.
Degryse B, Resnati M, Rabbani SA, Villa A, Fazioli F, Blasi F . Src-dependence and pertussis-toxin sensitivity of urokinase receptor-dependent chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. Blood 1999; 94: 649–662.
Nguyen DH, Webb DJ, Catling AD, Song Q, Dhakephalkar A, Weber MJ et al. Urokinase-type plasminogen activator stimulates the Ras/Extracellular signal-regulated kinase (ERK) signaling pathway and MCF-7 cell migration by a mechanism that requires focal adhesion kinase, Src, and Shc. Rapid dissociation of GRB2/Sos-Shc complex is associated with the transient phosphorylation of ERK in urokinase-treated cells. J Biol Chem 2000; 275: 19382–19388.
Smith HW, Marra P, Marshall CJ . uPAR promotes formation of the p130Cas-Crk complex to activate Rac through DOCK180. J Cell Biol 2008; 182: 777–790.
Shi Z, Liu Y, Johnson JJ, Stack MS . Urinary-type plasminogen activator receptor (uPAR) modulates oral cancer cell behavior with alteration in p130cas. Mol Cel Biochem 2011; 357: 151–161.
Osherov N, Levitzki A . Epidermal-growth-factor-dependent activation of the src-family kinases. European Journal of Biochemistry / FEBS 1994; 225: 1047–1053.
Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC et al. EGF receptor signaling stimulates Src kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 1999; 96: 677–687.
Cooper JA, MacAuley A . Potential positive and negative autoregulation of p60c-src by intermolecular autophosphorylation. Proc Natl Acad Sci USA 1988; 85: 4232–4236.
Rich JN, Reardon DA, Peery T, Dowell JM, Quinn JA, Penne KL et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol 2004; 22: 133–142.
Franceschi E, Cavallo G, Lonardi S, Magrini E, Tosoni A, Grosso D et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Br J Cancer 2007; 96: 1047–1051.
Lu-Emerson C, Norden AD, Drappatz J, Quant EC, Beroukhim R, Ciampa AS et al. Retrospective study of dasatinib for recurrent glioblastoma after bevacizumab failure. J Neurooncol 2011; 104: 287–291.
Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C et al. Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS ONE 2013; 8: e56505.
Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci USA 1994; 91: 7727–7731.
Sato K . Cellular functions regulated by phosphorylation of EGFR on Tyr845. Int J Mol Sci 2013; 14: 10761–10790.
Piwnica-Worms H, Saunders KB, Roberts TM, Smith AE, Cheng SH . Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell 1987; 49: 75–82.
Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther 2007; 6: 1167–1174.
Chan WC, Nie S . Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998; 281: 2016–2018.
Mukasa A, Wykosky J, Ligon KL, Chin L, Cavenee WK, Furnari F . Mutant EGFR is required for maintenance of glioma growth in vivo, and its ablation leads to escape from receptor dependence. Proc Natl Acad Sci USA 2010; 107: 2616–2621.
Nguyen D, Catling A, Webb D, Sankovic M, Walker L, Somlyo A et al. Myosin light chain kinase functions downstream of Ras/ERK to promote migration of urokinase-type plasminogen activator-stimulated cells in an integrin-selective manner. J Cell Biol 1999; 146: 149–164.
Kjoller L, Hall A . Rac mediates cytoskeletal rearrangements and increased cell motility induced by urokinase-type plasminogen activator receptor binding to vitronectin. J Cell Biol 2001; 152: 1145–1157.
Ma Z, Thomas KS, Webb DJ, Moravec R, Salicioni AM, Mars WM et al. Regulation of Rac1 activation by the low density lipoprotein receptor-related protein. J Cell Biol 2002; 159: 1061–1070.
Jo M, Takimoto S, Montel V, Gonias S . The urokinase receptor promotes cancer metastasis independently of urokinase-type plasminogen activator in mice. Amer J Pathol 2009; 175: 190–200.
Eastman BM, Jo M, Webb DL, Takimoto S, Gonias SL . A transformation in the mechanism by which the urokinase receptor signals provides a selection advantage for estrogen receptor-expressing breast cancer cells in the absence of estrogen. Cell Signal 2012; 24: 1847–1855.
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 2007; 21: 2683–2710.
Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ . A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res 1996; 56: 5079–5086.
Besnard-Guerin C, Newsham I, Winqvist R, Cavenee WK . A common region of loss of heterozygosity in Wilms' tumor and embryonal rhabdomyosarcoma distal to the D11S988 locus on chromosome 11p15.5. Hum Genet 1996; 97: 163–170.
Inda M, Bonavia R, Mukasa A, Narita Y, Sah D, Vandenberg S et al. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes Dev 2010; 24: 1731–1745.
Wykosky J, Mukasa A, Furnari F, Cavenee WK . Escape from targeted inhibition: the dark side of kinase inhibitor therapy. Cell Cycle 2010; 9: 1661–1662.
Jo M, Thomas K, Marozkina N, Amin T, Silva C, Parsons S et al. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem 2005; 280: 17449–17457.
Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS . Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene 2003; 22: 392–400.
Zhang S, Yu D . Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci 2012; 33: 122–128.
Aguirre Ghiso JA, Kovalski K, Ossowski L . Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol 1999; 147: 89–104.
Carriero MV, Franco P, Votta G, Longanesi-Cattani I, Vento MT, Masucci MT et al. Regulation of cell migration and invasion by specific modules of uPA: mechanistic insights and specific inhibitors. Curr Drug Targets 2011; 12: 1761–1771.
Montuori N, Cosimato V, Rinaldi L, Rea VE, Alfano D, Ragno P . uPAR regulates pericellular proteolysis through a mechanism involving integrins and fMLF-receptors. Thromb Haemost 2013; 109: 309–318.
Carriero MV, Del Vecchio S, Capozzoli M, Franco P, Fontana L, Zannetti A et al. Urokinase receptor interacts with alpha(v)beta5 vitronectin receptor, promoting urokinase-dependent cell migration in breast cancer. Cancer Res 1999; 59: 5307–5314.
Yu W, Kim J, Ossowski L . Reduction in surface urokinase receptor forces malignant cells into a protracted state of dormancy. J Cell Biol 1997; 137: 767–777.
Ma Z, Webb D, Jo M, Gonias S . Endogenously produced urokinase-type plasminogen activator is a major determinant of the basal level of activated ERK/MAP kinase and prevents apoptosis in MDA-MB-231 breast cancer cells. J Cell Sci 2001; 114: 3387–3396.
Alfano D, Franco P, Vocca I, Gambi N, Pisa V, Mancini A et al. The urokinase plasminogen activator and its receptor: role in cell growth and apoptosis. Thromb Haemost 2005; 93: 205–211.
Alfano D, Iaccarino I, Stoppelli M . Urokinase signaling through its receptor protects against anoikis by increasing BCL-xL expression levels. J Biol Chem 2006; 281: 17758–17767.
Ellis V, Behrendt N, Dano K . Cellular receptor for urokinase-type plasminogen activator: function in cell-surface proteolysis. Methods Enzymol 1993; 223: 223–233.
Parker JJ, Dionne KR, Massarwa R, Klaassen M, Foreman NK, Niswander L et al. Gefitinib selectively inhibits tumor cell migration in EGFR-amplified human glioblastoma. Neuro Oncol 2013; 15: 1048–1057.
Mazar AP, Ahn RW, O'Halloran TV . Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des 2011; 17: 1970–1978.
Jo M, Lester R, Montel V, Eastman B, Takimoto S, Gonias S . Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem 2009; 284: 22825–22833.
Acknowledgements
This work was supported by NIH R01 CA169096 (to SLG), R01 NS080939 (to FBF) and the Defeat GBM Research Collaborative, a subsidiary of National Brain Tumor Society (to WKC and FBF). WKC is a Fellow of the National Foundation for Cancer Research. We would like to thank Aran Merati and Nancy Du for their technical assistance with some of the experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Hu, J., Muller, K., Furnari, F. et al. Neutralizing the EGF receptor in glioblastoma cells stimulates cell migration by activating uPAR-initiated cell signaling. Oncogene 34, 4078–4088 (2015). https://doi.org/10.1038/onc.2014.336
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.336
This article is cited by
-
High expression of Bruton’s tyrosine kinase (BTK) is required for EGFR-induced NF-κB activation and predicts poor prognosis in human glioma
Journal of Experimental & Clinical Cancer Research (2017)